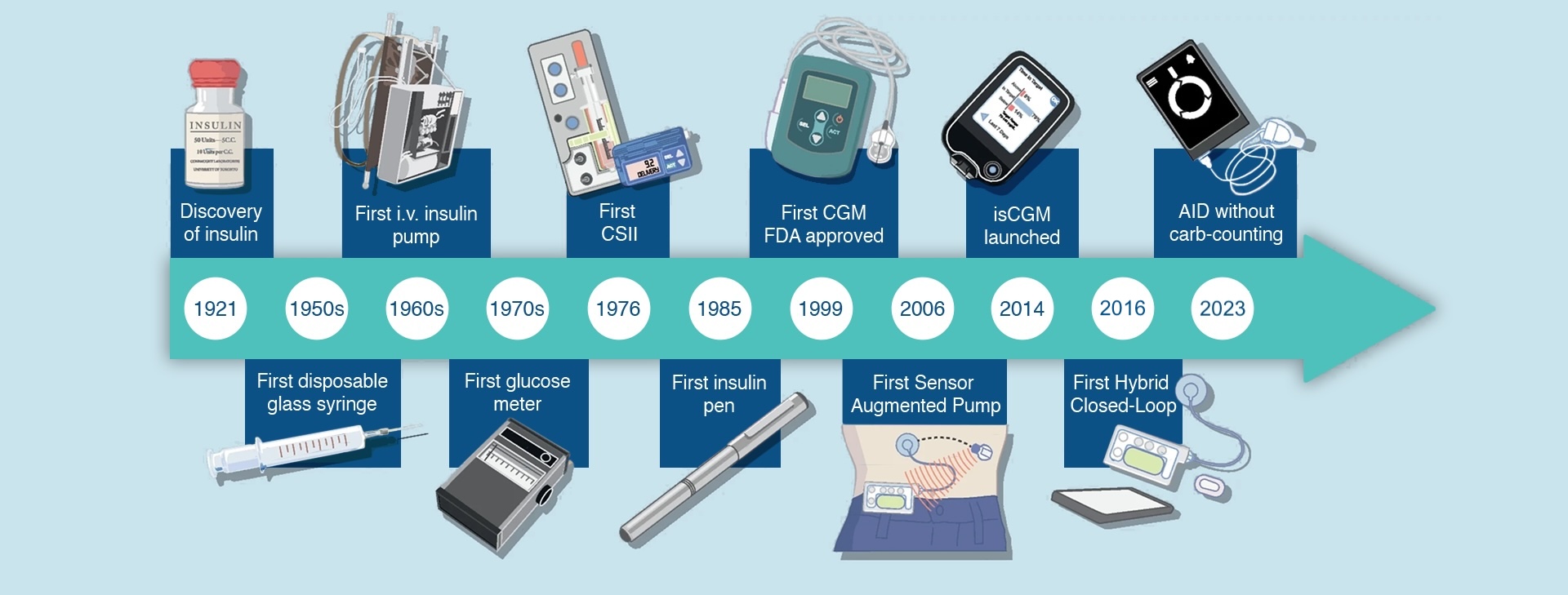
Portal DiabetesTM: developing the first artificial pancreas
Portal is developing the first artificial pancreas, a fully automated insulin delivery system, made possible by the fast pharmacokinetics of insulin delivered to the intraperitoneal space, an implantable pump, heat stable insulin, and modern continuous glucose monitor (CGM) technology. The Portal PumpTM is anticipated to deliver an average time in range of about 90 percent while relieving patients of the relentless management burden. Patients will no longer need to provide carbohydrate counts, meal announcements, exercise announcements, or wake in the night to manage their blood glucose. By restoring the function of a pancreas, Portal Pump TM empowers patients to effortlessly achieve excellent health outcomes.